Cornerstone Christian Homeschoolers’ Students Design Infant Incubators Using POETS RET-Developed Curriculum
January 24, 2019

Joe Muskin chats with Cornerstone Christian Homeschoolers about their incubator design.

Cornerstone Christian Homeschoolers test a number of chemical reactions to determine which will work best for their incubator.

Students measure the temperature as ice changes states from solid to liquid to gas. The thermometer readings are then transferred to a computer application that makes a line graph charting its changes over time
Over the last several months, 7th through 12th grade students who are a part of a home school support group, Cornerstone Christian Homeschoolers, have not only been learning some things about engineering and heat, but they have been discovering that engineers work to solve real-world problems. Using a POETS RET-developed curriculum, Joe Muskin, Education Coordinator for the NSF-funded POETS (Power Optimization for Electro-Thermal Systems) Engineering Research Center, has been working with the students who, after learning some of the science and engineering they might need to draw on, have been designing infant incubators for the developing world.
“One of the real problems of the world is that when babies are born prematurely, they can't regulate their body temperatures very well,” Muskin explains. “We in the Western world, we have incubators which solve the problem. So we think what we need to do then is ship off our old incubators. And we do. But the problem is they're sitting in the hospital unused because the people that need them are not at the clinics.”
“So what these students are doing is trying to come up with a real solution to this problem,” Muskin explains.
The idea is to use chemical reactions that produce a certain level of heat that can be sustained for a long period of time. So through the curriculum, the students have been exploring a number of different chemical reactions.
For example, while exploring chemical reactions, they’ve learned what's actually happening, how the molecules actually change. “We looked at what was going on,” Muskin says, “and we wondered, ‘Where did this gas come from? Why was it not here before?’ We did a lot of thinking, and we actually came up with the idea that this must be something new and different.”
Using inquiry-based learning techniques, Muskin reports that the students themselves have been “kind of driving that learning,” and, through exploration, have come up with a number of scientific principles on their own.
“They did, they figured out that there's something new here...something that wasn't here before. It's not just released; it's new. So they got that idea, and I think it makes it a lot more understandable. Not only that…we talked about ‘What is heat?’ And they came up with the idea that the molecules are moving at different speeds, and they're moving slowly when it's cold, and when you heat something up, they're moving fast.”

Joe Muskin and students watch as chocolate changes from a solid to a liquid, measuring the temperature changes over time. Once the experiment was done, they got to eact the fondue.

A student watches as ice melts much more slowly on the insulator material on the right.
In the context of designing and building their incubator, one of the problems the students ran into was that chemical reactions produce a lot of heat very suddenly, and then cool off really quickly. However, for an incubator to work properly, the heat needs to be long-term, and to somehow be captured.
To solve this issue, students needed to learn about another scientific principle: phase change. The idea was to try to make the heat more long term by using phase change materials, and thinking about what's going on when something is changing phase. Before building their incubators, they also needed to address the difference between a conductor and an insulator. So they studied things that conduct vs. things that insulate, not just heat, but electricity, and what's actually going on in these materials.

Students enjoy the fondue once the chocolate experiment is done.
One experiment they did involved placing ice cubes on two different surfaces, one a conductor, and one an insulator. In the image to the right, the surface on the right was the insulator, and the ice melted much more slowly.
“So all through this, they're actually learning a lot of science as they're trying to solve a real problem,” Muskin says. He adds that working to solve a real-world problem is actually really important so the students understand that “Engineering is not just a weird, meta thing; they’re actually solving a real problem, and they're actually saving lives. They really bought in to the idea of trying to save these infants.”
So the curriculum not only addresses a real problem that needs an understanding of the content that they want to teach, but it’s also being used as a model for why kids might want to consider engineering as a career. Plus, one thing that teachers who use the curriculum will appreciate, it’s aligned well with the science learning standards about heat and chemical reactions. “As they're doing this,” Muskin says of the students, “they may not realize it, but they're learning a whole lot of this content. So that's the purpose around this.”
The idea of trying to solve a real-world problem seems to appeal to thirteen-year-old Libby Boyer, an 8th grader. She says that was one of her favorite parts of the project: “It's cool having the idea of helping real-life problems,” then adds, “And I also like the chemical reacting kind of thing.”
Regarding potential discoveries she and her teammates had made, she explains, “We have some things that went around the temperature of what we need, which is good.” Their secret formula? Boyer and her team settled on a chemical reaction comprised of 17 grams of water, 17 grams of road salt, and 30 grams of Vaseline gel.
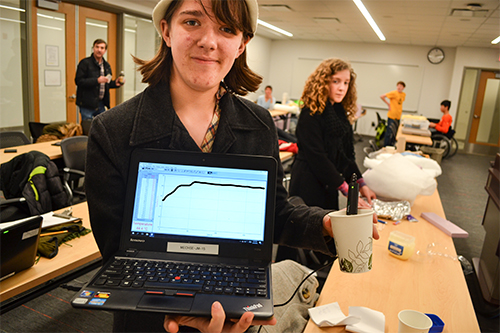
A student shows the data they recorded from their test chemical reaction, which quickly reached a peak, then maintained that temperature over a long period of time.

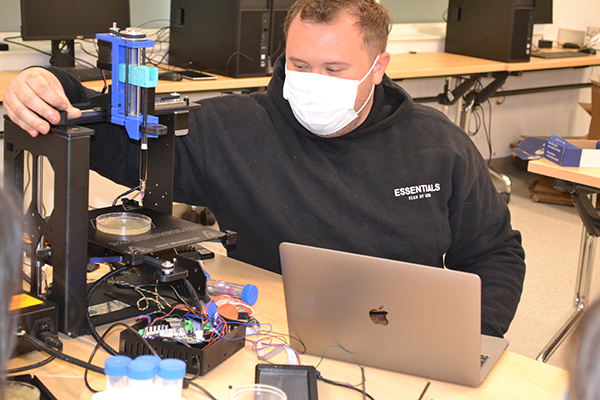
A student making his incubator prototype.
Like Boyer, 9th grader Matthew Lugardo reports that he also finds actually creating an incubator that might make a difference for people to be quite rewarding. “If we do make an improvement, this will be able to impact lives.” He adds that he finds this aspect really exciting, adding that last year Cornerstone did POETS’ solar car curriculum. “Being green is great,” he acknowledges, “but it's also really exciting to be able to do that [make a difference in peoples’ lives].
Lugardo appreciates Cornerstone’s collaboration with Muskin and their exposure to the POETS curricula, calling it “great, because we do so many different things. Whereas in perhaps a school club, I'd only be doing robotics or something. But in Cornerstone, we do fun things...like this, and last year we made solar cars and we also learned how to use CAD.”
Some things he’s learned through Cornerstone’s project with Muskin? “We've learned some stuff about chemical reactions, we learned about the problems that people face to distribute incubators in Africa with not enough electricity, and the infrastructure not being able to provide electricity to the people who need incubators. And we've learned stuff for how to use this program and more about conducting experiments.”
Regarding Muskin’s inquiry-based-learning instructional style, he adds, “I definitely like how hands on it is, and how it's not just us standing and watching a PowerPoint slideshow.”
Muskin comments on his work with groups like Cornerstone: “I think it's really important to reach all students. I think home school students are a group that tends to be overlooked.” Muskin adds that the teachers who are helping to develop the curriculum are actually testing the unit out in a school that’s in a rural area as well.
Story and photographs by Elizabeth Innes, Communications Specialist, I-STEM Education Initiative.
More: 6-8 Outreach, 8-12 Outreach, POETS, RET, 2019
For additional articles about POETS, see:
- Dr. Howard Fourth Graders Learn Engineering, Problem-Solving, While Building Solar Cars
- POETS’ Education Program Introduces Students of All Ages to Interdisciplinary Research in Electro-Thermal Systems
- POETS Seeks to Change the Attitudes, Shape of Students in the STEM Pipeline
- POETS, New NSF Center at Illinois, Poised to Revolutionize Electro-Thermal Systems

Two Cornerstone Christian Homeschoolers testing different chemical reactions watch the temperature trajectory of each on a computer.

Two students show off part of their incubator prototype.
 Joe Muskin sets up an experiment for the students, who are measuring the data for how long it takes water to change states from ice (a solid), to a liquid, then to a gas.
Joe Muskin sets up an experiment for the students, who are measuring the data for how long it takes water to change states from ice (a solid), to a liquid, then to a gas. 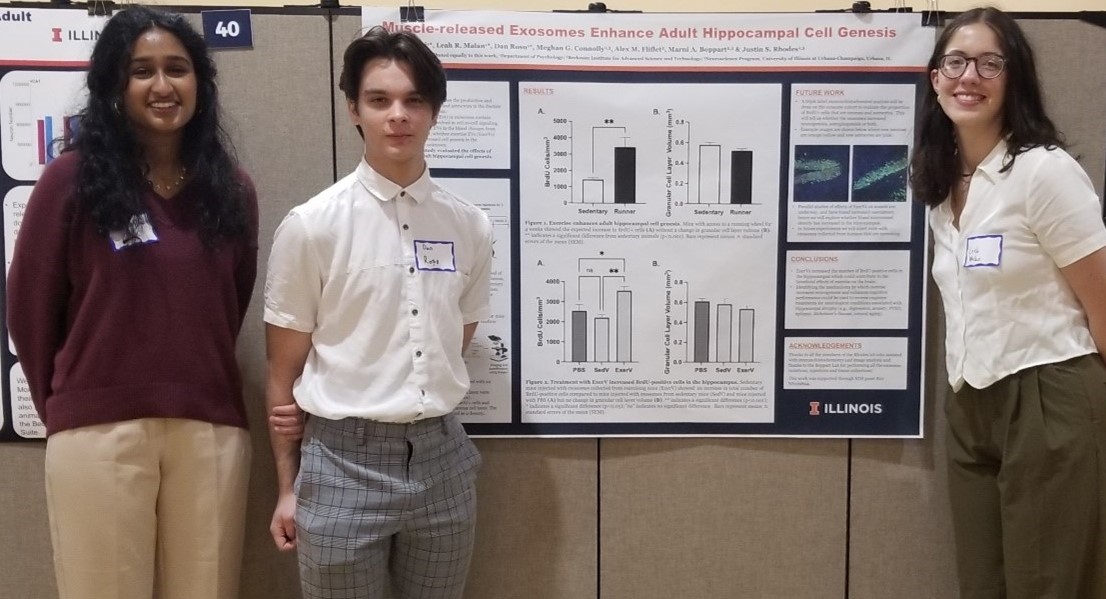










.jpg)