Van der Veen Team Teaches STEAM Studio Students Why Air Pressure Variations and Vacuums Warrant Wacky Weather

A STEAM studio youngster prepares in case the balloons pop as the air pressure is being sucked out of the bell jar.
What happens to balloons in a bell jar when you remove the air pressure and create a vacuum? What happens to marshmallows? The liquid in a barometer? How do these relate to our weather?
May 3, 2018
A number of STEAM Studio third–fifth graders discovered the answers to these questions and more when two PhD students from Chemistry Professor Renske van der Veen’s lab visited on Wednesday, April 25th and Friday, April 27th. Because the goal of Next Generation Schools’ after-school program is to emphasize STEAM (Science, Technology, Engineering, and Math [STEM], along with Art), Tyler Haddock and Ryan Cornelius dropped by to present some scientific demos about air pressure—and how these different air pressure and vacuum effects are related to the weather—as part of STEAM Studio’s Wacky Weather Week.
Why bring in experts to do demos? “Weather is not something that we can go outside and manipulate,” explains STEAM Studio Director Angela Nelson. “We have to wait for a sunny day and then a rainy day to see how the pressure has changed. With demonstrations like this, we can create a model that recreates these conditions and allow students to observe the outcomes all in one class period.”
So students were exposed to some weather-related experiments when Haddock and Cornelius used vacuum pumps to remove the air from some bell jars to determine what would happen to different objects placed inside the jars. But first, students received a work sheet, and prior to each experiment, were to develop a hypothesis explaining what they thought would happen in each experiment then report the results on the sheet once the activity was over.

A student develops multiple hypotheses for various scenarios involving behavior in vacuums.
Take the balloon experiment. Some of the kids (including this reporter), thought the balloons would shrink. Wrong! As the air was removed from the container, they grew. Why? Because as the number of air molecules in the jar decreased and no longer pressed on the outside of the balloon, the molecules inside the balloon remained and continued to press on the inside surface of the balloon, causing the balloon to expand in size. In fact, the balloons soon took the shape of the bell jar.
In another experiment, a barometer was constructed inverting a tube filled with colored water in a small dish of water. When it was placed inside the bell jar and the air was removed, how would the water level in the tube change? It dropped! As air was removed, it could no longer press down on the water in the dish. This lack of pressure allowed the level in the tube to decrease by its own weight.

STEAM Studio Director Angela Nelson waits to see what will happen to the barometer as the air pressure is removed from the bell jar.
What happened to marshmallows as air pressure was removed from the bell jar? First they expanded as the air was being sucked out of the bell jar—just like the balloon. But then, after the air was let back into the chamber, they got smaller again. What was happening? Because marshmallows have a lot of air pockets inside, as the air was removed from the container, there was less air pressure on the outside of the marshmallow than on the inside, so the inside air could push its way out very easily, making the marshmallows get bigger. But once all the air returned, the marshmallows shriveled back to their original size.

Tyler Haddock (left) and a STEAM Studio student watch as two marshmallows expand as a vacuum is created in the bell jar.
Another experiment involved seeing which would fall faster, a feather or a paper clip. Outside of the bell jar, the paper clip won the race. Inside the bell jar, however, the two fell at the same rate, because with no air resistance to push against the feather to slow it down, it fell at the same rate as the clip. This is verification of Galileo’s experiment. He supposedly dropped objects of different mass from the Tower of Pisa and observed them fall at the same speed!
The final experiment involved boiling water. Can water boil with no heat applied? The students first learned the definition of boiling, which might be a bit different than one expects: It’s when liquid turns into a vapor.
Another thing students learned was that water boils at different temperatures depending on air pressure. For example, the higher you go in the atmosphere, the lower the temperature at which water boils, because there’s less air pressure. So as the pressure was lowered in the vacuum chamber, the water began to boil, and contrary to popular opinion, the temperature remained constant.

Chemistry PhD student Ryan Cornelius interacts with a STEAM Studio student regarding what might happen with the balloon when the air pressure is removed.
Through these demonstrations that allowed students to see with their own eyes scientific principles in action, Nelson and her teachers were able to “take the more abstract concept of atmospheric pressure and make it meaningful for the students,” she explains. By encouraging students to explore how vacuums work and explore what happens to a barometer as the pressure decreases, Nelson adds:
“The activity also went past the scope and sequence of our weather unit and explored the relationship of pressure in a range of different fields and real-world experiences It allowed the students to explore the microscopic world of chemistry through molecules that cause the macroscopic weather changes that we are discussing around the world.”

A STEAM Studio student discusses her hypothesis regarding what she thinks will happen with the balloons when the air pressure is removed.
Nelson adds that her students learned more than just how the air pressure/vacuum effects impact weather. The experiments allowed her students to see multiple uses for the same technology—vacuums in space, how objects fall, Boyle’s law, and the impact of pressure on boiling temperature. “This allows students to break the misconception that a science concept only applies to one situation and opens the door for creativity and awareness of the multiple applications of a scientific concept,” she explains.
Haddock, agrees with Nelson as to the benefit of their activities for her students: “Abstract concepts in science are hard to grasp at a young age,” he admits. “Seeing the balloons expand or the barometer level drop gives much more weight to the explanation of how. I could really see the gears turning as the students were asked to conclude why they observed what they did. Seeing the ‘Eureka!’ moment for several students is very encouraging.”

Tyler Haddock prepares to drop a feather and a paper clip to see which falls faster outside of the bell jar; the same test was then conducted inside a bell jar with the air pressure removed.
He also reports he believes that the students benefitted not just by learning about science, but by interacting with the grad students. “Young students look up to college and graduate age students as mentors and role models,” he says, “Getting to work one-on-one with ‘big kids’ can be an inspiration for students to make their own path as scientists.”
But it wasn’t just the younger students who benefitted from the encounters; Haddock indicates that the interaction between grad students and youngsters goes both ways: “As a graduate student, it’s very refreshing to answer the creative questions asked as they wonder at the science of the demos. I usually have forgotten when and where I learned principles like vacuum, so to see the learning process and inquisition of the students firsthand is exciting.”
Nelson explains why she encourages guest experts like van der Veen’s grad students to visit her program. “It allows students to interact with experts in the field and learn how research is conducted in a multitude of fields,” she explains. “It also allows us to provide authentic experiences using equipment and resources that would not normally be available to us. Students also learn how to communicate and share their experiences with scientists.”
Even beyond the knowledge the students gained, and their exposure to the graduate students, Nelson believes the activities fostered a spirit of inquiry amongst her students, pushing them to “ask questions and search for answers.” Plus, she believes having enthusiastic experts on hand to help students hunt for answers inspires them to ask more questions. In fact, Nelson believes it’s meaningful for students to hear the experts admit, “‘I’m not sure what would happen. I have a theory, let’s test it!’ Knowing that you do not have to know it all and being open to discovering it is an integral part of being a scientist and can sometimes be lost to young scientists,” says Nelson. “These opportunities remind them of that process.”
STEAM Studio Director Angela Nelson shares how the partnership with Professor van der Veen, who’s affiliated with both Illinois’ Department of Chemistry and Materials Science and Engineering Department, came about. Van der Veen had heard about STEAM Studio and reached out to Nelson to discuss potential collaboration opportunities. Based on van der Veen’s expertise with vacuums and previous experience doing vacuum-related demos for local schools, as well as STEAM’s upcoming units, they determined that activities about atmospheric pressure seemed like a perfect fit.
While the visit was beneficial for the youngsters—it was actually a win-win for both groups, because it gave van der Veen’s team a chance to test run their outreach material. Van der Veen’s early 2018 NSF CAREER award helped to make these experiments possible for the students. The grant allocated funding for the purchase of pressure chambers to be used in a community outreach component in the Champaign-Urbana area. So van der Veen and Haddock had been developing vacuum demos and experiments since last summer.
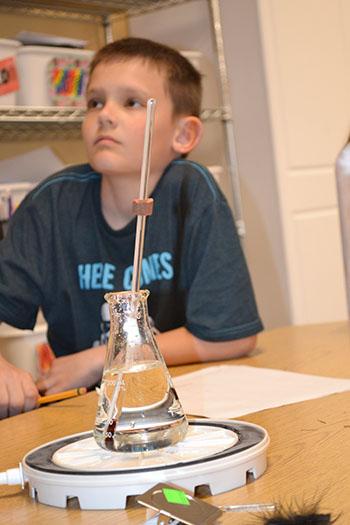
Participants learn how water can be boiled by simply lowering the air pressure in a bell jar without applying heat
Haddock says, “STEAM was a perfect place to do our first demos given the relevance of Wacky Weather Week, the way science is taught in an engaging environment, and the familiarity of the students with concepts related to the demos—like pressure.”
Tyler Haddock is a 2nd year Chemistry PhD student who uses X-ray and laser pulses of a trillionth of a second in duration to study how materials change when they interact with light. Ryan Cornelius, a first-year Chemistry PhD student who is also in the van der Veen research group, works on an electron microscope (which uses electrons instead of light) and is interested in studying how light changes the shape of materials within the microscope.
Nelson expresses her thanks to van der Van’s team: “STEAM Studio is thankful for the time and energy that Dr. Van der Veen’s lab put into creating this wonderful opportunity.”
Story and photographs by: Elizabeth Innes, Communications Specialist, I-STEM Education Initiative
More: K-6 Outreach, Next Generation School, STEAM/SciArt, STEAM Studio, 2018
For more I-STEM articles about STEAM Studio and Next Generation School, please see the following:
- STEAM Studio Uses Science, Technology, and Art to Go “Virtually Spelunking” in Caves—Exploring Everything From Spiders to Bats to 3D Cave Painting to GPS
- Wai-Tat Fu's Lab Partners with STEAM Studio To Make STEM, Spatial Reasoning Fun
- STEAM STudio's STEAMcation Students Visit RailTEC...Learn All About Trains
- Illinois' MCBees Expose STEAM Studio's STEAMcation Students to Medieval Science
- Next Gen's STEAM Studio: An After-School STEM Program With a Dab of Creativity

Two students watch to see how fast a feather and a paper clip fall in a vacuum.










.jpg)