Campers Experience Ooey-Gooey Science
August 17, 2012

Don Decoste explains the properties of liquid nitrogen to the campers.
School isn't the only place youngsters can learn about science. Kids who experienced Indian Acres Day Camp's Ooey Gooey Science Week in July discovered that you can use ordinary, everyday things most people have at home to do science experiments—some ooey gooey, but all of them fun!
According to Laura Bruce, a team leader at Indian Acres day camp, whose role is to plan and lead activities for the campers in the 5-6-year-old group, "Without even knowing it, children experience science every day. Ooey Gooey Science Week provides campers with opportunities to explore a special kind of science—ooey gooey science!"
Anna Rice, the camp director, reported that the team leaders chose to do "Ooey Gooey Week" because they thought campers would "enjoy learning about science in a fun, hands-on learning environment...children learn best when they can get their hands dirty and be active particpants in the learning process."
Some of the week's ooey gooey activities included making and playing with all sorts of "slime." For example, campers made oobleck. Named after the fictional green goo in the Dr. Seuss book, Bartholomew and the Oobleck, the slimy stuff is made by adding water to cornstarch. Oobleck has very unusual properties. For instance, if you grab a handful, it will act hard like a solid; however, if you quit applying pressure, it will ooze out of your hand like a liquid. The youngsters also made and played with other kinds of goo: oatmeal play dough (made of flour, water, and oatmeal) as well as salt play dough (made of salt, flour, and water).

"Naked" egg.
Campers also conducted mini chemistry experiments with acids and bases. For example, they used vinegar and eggs to make "naked" eggs, or eggs without shells. In this experiment, an egg is placed in a container and covered with vinegar for about 48 hours. This contact with the vinegar causes the eggshell to dissolve and bubbles to be released. What is left is an egg in the membrane only, and because the egg no longer has a shell, it is translucent. What is the science behind it? It's an acid-base reaction. The acid in the vinegar causes the solid calcium carbonate crystals that make up the eggshell (a base) to break apart into calcium and carbonate. The calcium ions float free, while the carbonate is released as carbon dioxide—the bubbles that you see.

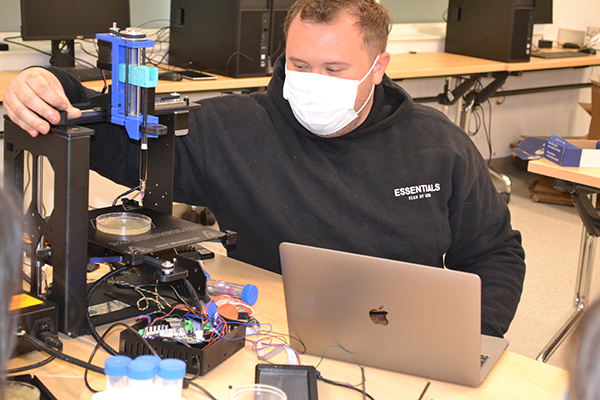
Youngsters are engaged during the chemistry presentation by Don Decoste & Jesse Miller at Indian Acres Camp.
Campers also played with another base and acid: baking soda and vinegar. The campers discovered that when the two were mixed together, it created bubbles. What's the science behind the fun? The vinegar (an acid) reacts with the baking soda (a base), forming a gas (carbon dioxide). The bubbles are filled with carbon dioxide.
They also made pop geysers using diet cola and Mentos candy mints, and here's the science behind it: the gum arabic, what makes Mentos chewy, breaks down the surface tension that holds the water molecules together, releasing a lot of gas! Campers even worked on making bubbles big enough to sit inside, and created squish paintings while mixing new colors.
However, the pièce de résistance of the week was the visit by Illinois Chemistry Department's gurus of goo, Donald Decoste and Jesse Miller, who performed a combination comedy routine and magic act involving science. Director Anna Rice had seen Don on the Channel 3 morning show and thought he could "engage the campers and spark their interest in the field of science."
Decoste and Miller's act incorporated some of the more showy aspects of chemistry—most of it involving some kind of smoke or vapor. Some of the experiments involved balloons. They startled the audience with a loud pop and flames as they ignited a gas-filled balloon. They entertained their audience by alternately taking swigs from some other balloons; Don took a breath from a helium-filled balloon, which made his voice shoot up a register to sound like one of the chipmunks, while Jesse breathed in gas from a Sulfur-Hexafluoride-filled balloon, which caused his to boom out in a lower-than-normal register like Darth Vadar.

Jesse Miller sneaks more liquid detergent into the container.
Another experiment involved sending an electric current into a dill pickle causing it to smoke and glow as the electricity excited the electrons in the sodium atoms.
The two did a lot with liquid nitrogen. For instance, they quick-froze rubber racket balls in liquid nitrogen, then dropped them on the floor, where they shattered into fragments.
But the show-stopping finale was such a large production number that it had to be taken outside. In this comedy routine also involving liquid nitrogen and dish soap, Don, who played the straight man, repeatedly stressed how much he could trust his long-time colleague to put only a dab of dish soap into the container. Meanwhile, Jesse, with a number of winks and asides to the audience, proceeded to dump in practically the whole bottle. To the delight of the campers, once Don added the liquid nitrogen, it erupted in a huge geyser of smoke-like vapor and suds, half of which ended up on Don. Jesse, of course, had retreated to a safer distance.
All in all, it appears that the experience was not only educational, but fun...just what the camp leaders were wanting. According to Rice, the impact they were hoping for was: "that the children had fun, and that they learned the science behind the experiments. We also hoped that they shared what they saw and learned with their families and friends and they choose to come to day camp again."
Did the kids learn anything about science during the week? While they might not remember the basics after their week-long encounter with goo, there's most likely one thing that will stick with them for quite a while: science doesn't have to be boring.
Article and photographs by Elizabeth Innes, Communications Specialist, I-STEM Education Initiative
More: Chemistry, K-6 Outreach, Summer Camp

Don Decoste pours liquid nitrogen into a container, causing an "eruption" of vapor and suds.










.jpg)